Aspartic acid
| Aspartic acid | |
|---|---|
 |
|
 |
|
|
Trivial: Aspartic acid
Systematic: 2-Aminobutanedioic acid |
|
|
Other names
Aminosuccinic acid, asparagic acid, asparaginic acid[1]
|
|
| Identifiers | |
| CAS number | 617-45-8 56-84-8 (L-isomer) 1783-96-6 (D-isomer) |
| PubChem | 424 |
| ChemSpider | 411 |
| EC-number | 200-291-6 |
|
SMILES
O=C(O)CC(N)C(=O)O
|
|
|
InChI
InChI=1/C4H7NO4/c5-2(4(8)9)1-3(6)7/h2H,1,5H2,(H,6,7)(H,8,9)
Key: CKLJMWTZIZZHCS-UHFFFAOYAE |
|
| Properties | |
| Molecular formula | C4H7NO4 |
| Molar mass | 133.1 g mol−1 |
| Hazards | |
| EU Index | not listed |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
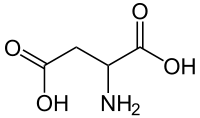
Aspartic acid (abbreviated as Asp or D; Asx or B represent either aspartic acid or asparagine)[2] is an α-amino acid with the chemical formula HO2CCH(NH2)CH2CO2H. The carboxylate anion of aspartic acid is known as aspartate. The L-isomer of aspartate is one of the 20 proteinogenic amino acids, i.e., the building blocks of proteins. Its codons are GAU and GAC.
Aspartic acid is, together with glutamic acid, classified as an acidic amino acid with a pKa of 4.0. Aspartate is pervasive in biosynthesis. As with all amino acids, the location of acid protons depends on the pH of the solution and the crystallization conditions.
Contents |
Discovery
Aspartic acid was first discovered in 1827 by Plisson, synthesized by boiling asparagine (discovered in 1806) with a base.[3]
Forms and nomenclature
The term "aspartic acid" refers to either of two forms or a mixture of two.[2] Of these two forms, only one, "L-aspartic acid", is directly incorporated into amino acids. The biological roles of its counterpart, "D-aspartic acid" are more limited. Where enzymatic synthesis will produce one or the other, most chemical syntheses will produce both forms, "DL-aspartic acid".
Role in biosynthesis of amino acids
Aspartate is non-essential in mammals, being produced from oxaloacetate by transamination. In plants and microorganisms, aspartate is the precursor to several amino acids, including four that are essential: methionine, threonine, isoleucine, and lysine. The conversion of aspartate to these other amino acids begins with reduction of aspartate to its "semialdehyde," O2CCH(NH2)CH2CHO.[4] Asparagine is derived from aspartate via transamidation:
- -O2CCH(NH2)CH2CO2- + GC(O)NH3+ O2CCH(NH2)CH2CONH3+ + GC(O)O
(where GC(O)NH2 and GC(O)OH are glutamine and glutamic acid, respectively)
Other biochemical roles
Aspartate is also a metabolite in the urea cycle and participates in gluconeogenesis. It carries reducing equivalents in the malate-aspartate shuttle, which utilizes the ready interconversion of aspartate and oxaloacetate, which is the oxidized (dehydrogenated) derivative of malic acid. Aspartate donates one nitrogen atom in the biosynthesis of inositol, the precursor to the purine bases.
Neurotransmitter
Aspartate (the conjugate base of aspartic acid) stimulates NMDA receptors, though not as strongly as the amino acid neurotransmitter glutamate does.[5] It serves as an excitatory neurotransmitter in the brain and is an excitotoxin.
Sources
Dietary sources
Aspartic acid is not an essential amino acid, which means that it can be synthesized from central metabolic pathway intermediates in humans. Aspartic acid is found in:
- Animal sources: luncheon meats, sausage meat, wild game
- Vegetable sources: sprouting seeds, oat flakes, avocado, asparagus, young sugarcane, and molasses from sugar beets.[1]
- Dietary supplements, either as aspartic acid itself or salts (such as magnesium aspartate)
- The sweetener aspartame (NutraSweet, Equal, Canderel, etc.)
Chemical synthesis
Racemic aspartic acid can be synthesized from diethyl sodium phthalimidomalonate, (C6H4(CO)2NC(CO2Et)2).[6]
References
- ↑ 1.0 1.1 "862. Aspartic acid". The Merck Index (11th ed.). 1989. p. 132. ISBN 091191028X.
- ↑ 2.0 2.1 "Nomenclature and symbolism for amino acids and peptides (IUPAC-IUB Recommendations 1983)", Pure Appl. Chem. 56 (5): 595–624, 1984, doi:10.1351/pac198456050595.
- ↑ R.H.A. Plimmer (1912) [1908]. R.H.A. Plimmer & F.G. Hopkins. ed. The chemical composition of the proteins. Monographs on biochemistry. Part I. Analysis (2nd ed.). London: Longmans, Green and Co.. p. 112. http://books.google.com/?id=7JM8AAAAIAAJ&pg=PA112. Retrieved January 18, 2010.
- ↑ Lehninger, Albert L.; Nelson, David L.; Cox, Michael M. (2000), Principles of Biochemistry (3rd ed.), New York: W. H. Freeman, ISBN 1-57259-153-6.
- ↑ Chen, Philip E.; Geballe, Matthew T.; Stansfeld, Phillip J.; Johnston, Alexander R.; Yuan, Hongjie; Jacob, Amanda L.; Snyder, James P.; Traynelis, Stephen F. et al. (2005). "Structural Features of the Glutamate Binding Site in Recombinant NR1/NR2A N-Methyl-D-aspartate Receptors Determined by Site-Directed Mutagenesis and Molecular Modeling". Mol. Pharmacol. 67 (5): 1470–84. doi:10.1124/mol.104.008185. PMID 15703381. http://molpharm.aspetjournals.org/cgi/content/full/67/5/1470..
- ↑ Dunn, M. S.; Smart, B. W. (1950), "DL-Aspartic Acid", Org. Synth. 30: 7, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV4P0055; Coll. Vol. 4: 55.
See also
- Aspartate transaminase
- Sodium poly(aspartate), a synthetic polyamide
- American Chemical Society (21 April 2010). "Ancestral Eve' Crystal May Explain Origin of Life's Left-Handedness". ScienceDaily. http://www.sciencedaily.com/releases/2010/04/100421121501.htm. Retrieved 2010-04-21.
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||